Clinical studies have been initiated worldwide to determine whether BCG vaccination contributes to the reduction of COVID-19 severity. In a previous blog, I hypothesized that BCG inoculation, mainly for classical acquired immunity, would act to suppress COVID-19. In this article, I would like to consider the immune mechanism from the aspect of boosting innate immunity memory based on relatively new literature.
Incidentally, this article is not a recommendation to try BCG in the general medical community for prevention of new coronas for purposes other than conventional tuberculosis prevention.
What is epigenetic?
The word "genetics" can be translated as “遺伝学”, but when you add "epi" to it, it means "after ~". In other words, if you force the word "epigenetic" into Japanese, it becomes “後成的遺伝上の”. The term epigenetic has been around for a long time, but the idea in the field of research has been generally active since around 2000.
Vertebrates and microorganisms, including humans, have cells with nuclei and DNA (deoxyribonucleic acid) stored in them. DNA molecules are usually distributed in the nucleus as fibrous structures wrapped around proteins called histones, and the genetic information is contained in DNA as a polymer.
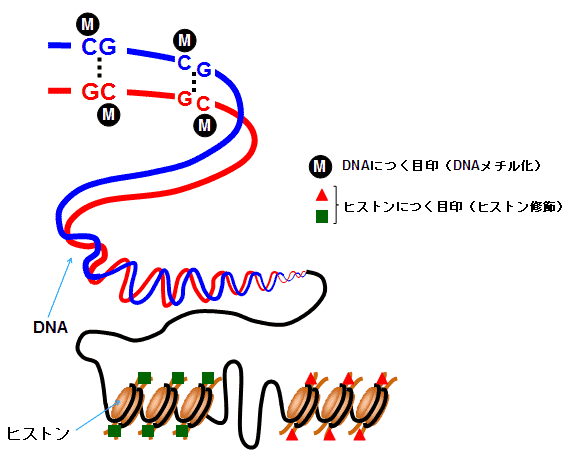
This DNA is something that every living organism is born with, and its genetic information is unchangeable if nothing else. However, when a certain part of DNA is methylated and marked/labeled, or a chemical decoration occurs in the surrounding protein, molecules and proteins corresponding to the gene are generated acquired. This is called an epigenetic change or gene expression. A chemically decorated genome is called an "epigenome". This gene expression is either up-regulated or down-regulated by acetylation or deacetylation of histones. Epigenetic reprogramming means that these DNA markers are erased and reconstituted. Epigenetic reprogramming is said to be involved not only in DNA methylation, but also in the regulation of RNA expression (microRNAs and long non-coding RNAs).
Dynamic innate immune memory
In the classical interpretation of immunology, immunity was divided into "innate immunity" and "acquired immunity", and the division of functions was roughly considered as follows.
- In "innate immunity," macrophages (Mφ), dendritic cells (DC), mast cells, and natural killer cells (NK cells) non-specifically phagocytose pathogens and, in some cases, present antigens that are characteristic of the pathogen.
- In "acquired immunity", T cells and B cells receive signals from the innate immune system and recognize the characteristics of invading pathogens to activate killer cells and mass produce antibodies to repel them. Some cells remain in memory of the pathogen's characteristics and prepare for the next response. (this is the vaccine's effect.)
Considering the above roles, it can be interpreted that it is in acquired immunity that pathogen characteristics are remembered, but recent studies have shown that memory is not only present in acquired immunity, but also in innate immunity. While the classical interpretation is that innate immunity is considered a relatively static response, in fact, it is increasingly thought to be a dynamic and interactive response occurring, such as remembering pathogen features or boosting innate immunity in a non-specific way to pathogens. Epigenetic reprogramming is said to be involved in the mechanism that causes such a response.
It is increasingly believed that BCG inoculation not only acquires immunity against Mycobacterium tuberculosis, but also strengthens a dynamic innate immune system that responds to more than just bacteria.
Let's organize a little prerequisite knowledge to explain this. Actors (mature cells) such as Mφ, DC, T cells, and B cells, which appear in immunity, are included in white blood cells and living tissues, but they did not originally exist independently. These cells are differentiated from their parent's cells, called progenitor cells.
For example, if we classify progenitor cells by Mφ and trace them back to their ancestors, we get the following
HSC (HSC)←Pluripotent progenitor cell (MPP)←granulocyte-macrophage progenitor cell (GMP)←granulocyte-macrophage-forming cell (CFU-GM)←macrophage colony-forming cell (CFU-M)←monocyte←Mφ
(Check out this site and the literature if you want to know more.)
The cytokines produced by each differentiation are various and complex. Putting aside the details, the point is that there are HSCs and MPPs in the bone marrow, and in the case of Mφ, these are the primary mature cells.
Now back to BCG. This paper shows that BCG (BCG Tice strain) stimulates HSCs and MPPs to produce epigenetically remodified Mφs in a mouse model (*1).
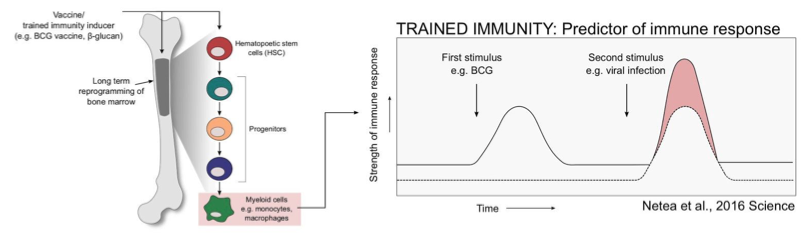
The proliferation of leukocyte cells produced from HSCs and their differentiated cells by vaccination involves stimulating factors such as pattern recognition receptors (PRRs), cytokines such as IFNγ, and G-CSF.
Since HSCs are self-renewing cells, if vaccines such as BCG indirectly leave some mark (epigenomic labeling) on HSCs that characterizes the differentiated cells, it is not surprising that BCG may have an innate immune memory against common pathogens. This paper shows that the up-regulated genes in both HSC and MPP were expressed in the BCG-inoculated mice compared to the non-BCG-inoculated mice. Interestingly, on the other hand, the genes involved in differentiation into lymphocytes were down-regulated. Mφs differentiated from HSCs are more protective in mice inoculated with BCG (using mice depleted of T cells in the bone marrow, just in case) against intentional infection by Mycobacterium tuberculosis. And such Mφs have been shown to have high gene expression levels that produce the pro-inflammatory cytokines IFNγ, Tnf, and IL-1β, and the expressed genes are H3K4me3 and H3K27ac. →(※A)
In another literature, these H3K4me3 and H3K27ac are also involved in the production of DCs from monocytes, independent of BCG. (*2)
This suggests that after the activation of innate immunity, DCs may be activated at the same time when Mφ is activated using these expressed genes as a key, and then quickly transition to acquired immunity through antigen presentation. →(※B)
H3K4me3 and H3K27ac represent methylation of histones and acetylation of histones, and H3K27ac represents acetylation of histones. This seems to indicate a state of being ready to have cell differentiation quickly activated/inhibited. (*3)
The details are beyond my comprehension, so I won't go into any more depth here. (Please let me know if anyone knows more.)
The above is not all the explanation for BCG enhancing innate immune cells such as Mφ, but it seems to explain one mechanism that cannot be derived from the classical interpretation.
The effect of BCG vaccination on the immune response
Mechanism of BCG inoculation in the Mφ
Since BCG is a bacterium that has weakened the tuberculosis bacteria, the immune response of BCG in the body should be similar to the internal mechanism of the tuberculosis bacteria when they are infected. Although the bacterium tuberculosis has been known for more than 100 years, its amazingly complex mechanisms in the body have only recently become known. For details, please refer to the literature here (*4), but here is a part of it. When common bacteria enter the body, they are eaten by phagocytic cells such as Mφ, which has a digestive organ (phagosome) similar to the stomach and is broken down when pathogens enter this digestive organ. However, the tuberculosis bacteria have the ability to multiply for a certain period of time in Mφ to prevent it from being digested by any means after being eaten by Mφ. Pathogens that enter the phagosome are usually degraded by enzymes contained in the lysosome to form the phagolysosome in the Mφ. In the case of Mycobacterium tuberculosis, however, LAM and metalloproteinase (Zmp1) produced by Mycobacterium tuberculosis inhibit the activation of caspase-1 in the Mφ, thereby inhibiting phagolysosomal fusion. Caspase-1 has the effect of processing the IL-1β precursor pro-IL-1β and making it ready to secrete IL-1β. (*5)
In this way, tuberculosis bacteria can survive in Mφ, and subsequently infected Mφ activates Mφ by producing cytokines such as TNF-α, IL-1, IL-12, and IL-18 by various mechanisms.
[added on May 27, 2020]
The uptake into the phagosome described above occurs by a mechanism called autophagy induction (*7). After the formation of autophagosomes and autolysosomes, the tuberculosis bacteria are degraded, but it is not known if they are antigen-presented after degradation. If antigen presentation occurs after tuberculosis bacillus degradation by Mφ or DC, there may be a common antigen after COVID-19 is degraded by a mechanism similar to that of the antigen. If this is correct, it would be conclusive evidence that BCG has an effect on COVID-19.
Influence on T-cell-associated infection defense responses
In experiments using mice, it has been shown that when mice are fed BCG, they produce cytokines and chemokines such as IFNγ and TFNα and activate inflammatory cells such as Mφ and neutrophils, and express PD-1L, a substance that binds specifically to PD-1. PD-1 is a receptor expressed on activated T cells and its gene, which was discovered in 1992 in Honjo's laboratory at Kyoto University. This paper shows that when PD-1L binds to the PD-1 receptor, the inhibitory signal inhibits Th1 type T cell function and suppresses the excessive inflammatory response. PD-1 is a substance that has been implicated in cancer immunotherapy, but we have not seen many papers on other types of pneumonia, so we may learn something by confirming the extent to which BCG vaccination status affects the expression of PD-1-related signals after a long period of time.
IL-1β in innate immunity
IL-1β is a cytokine often found in papers describing innate immunity, and in the context of BCG, this paper also describes IL-1β as playing an important role in training immunity. In fact, when monocytes in the test tube were pre-stimulated with IL-1β, they expressed the genes H3K4me3 and H3K27ac, which subsequently protected them from external viral infection. (*6)
Here again, a strong relationship between IL-1β and H3K4me3 and H3K27ac is evident.
Differences in innate immune enhancement by sub-strains
Here again, let's review the experimental results of the paper I mentioned in my previous blog. According to the paper, the cytokines produced when inoculated with the BCG Tokyo strain were IL-1α, IL-1β, IL-6, and IL-24.
If the BCG Tokyo strain has the most enhanced innate immunity of the sub strains, the most likely scenario would be that IL-1β would be the key. In other words, after the BCG Tokyo strain inoculation, IL-1β is produced in large numbers along with the mechanism of infection with Mφ, and gene expression occurs, resulting in a particularly enhanced innate immunity. In this case, not much antibody is produced. When humans are infected with the virus, Mφ is activated by the mechanism in (※A) to phagocytose and repel the virus. Even if it takes a long time to fight off the virus, the person does not become seriously ill because acquired immunity centered on T cells is quickly activated by the mechanism of (*B), and antibodies are produced to drive out the virus.
In other strains than BCG Tokyo, IL-1β is not so strongly activated but IFNγ is produced, acquired immunity may respond normally and resolve with moderate symptoms. When BCG is inoculated, it invades Mφ through the receptor called NOD2 in Mφ, but the amount of IL-1β secretion may be different due to the difference in toxicity between the sub-strains.
Of course, the mechanism of innate immunity (※A) does not describe anything about infection other than that of Mycobacterium tuberculosis, so I don't know if it applies to COVID-19. A similar verification can be confirmed in experiments with SARS-CoV-2 and mice. In addition to BCG Tokyo, it may be possible to express IL-1β for a longer period of time in sub-strains other than BCG Tokyo with the same level of virus protection. (I'd love it if someone could verify it.)
The above is a recent immunological perspective on the protection against COVID-19 by BCG vaccination. There is not much evidence on the memory of innate immunity, and I feel that it is still necessary to investigate the mechanisms of gene expression and its long-term maintenance during differentiation from HSC to mature cells. Considering this, we may be in a time when a paradigm shift from the classical interpretation of acquired immunity to an additional perspective of innate immunity may be needed.
【References】
(※1) Eva Kaufmann et al, BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis, 2017
(※3) The Epigenetic Basis of Hematopoietic Stem Cell Aging
(※4) Regulation of the expression of host infection defense by Mycobacterium tuberculosis (in Japanese)
(※5) J. F. Foley, A Calming Touch. Sci. Signal. 2, ec233 (2009).
Translated with www.DeepL.com/Translator (free version)